Complex Mechanism of the Gas Phase Reaction between Formic Acid and Hydroxyl Radical. Proton Coupled Electron Transfer versus Radical Hydrogen Abstraction Mechanisms | Journal of the American Chemical Society

SOLVED: The value of Ka for formic acid HCOOH is 1.80x10^-4. Write the equation for the reaction that goes with this equilibrium constant: (Use H2O instead of H+.)

Electronically excited states of formic acid investigated by theoretical and experimental methods - ScienceDirect
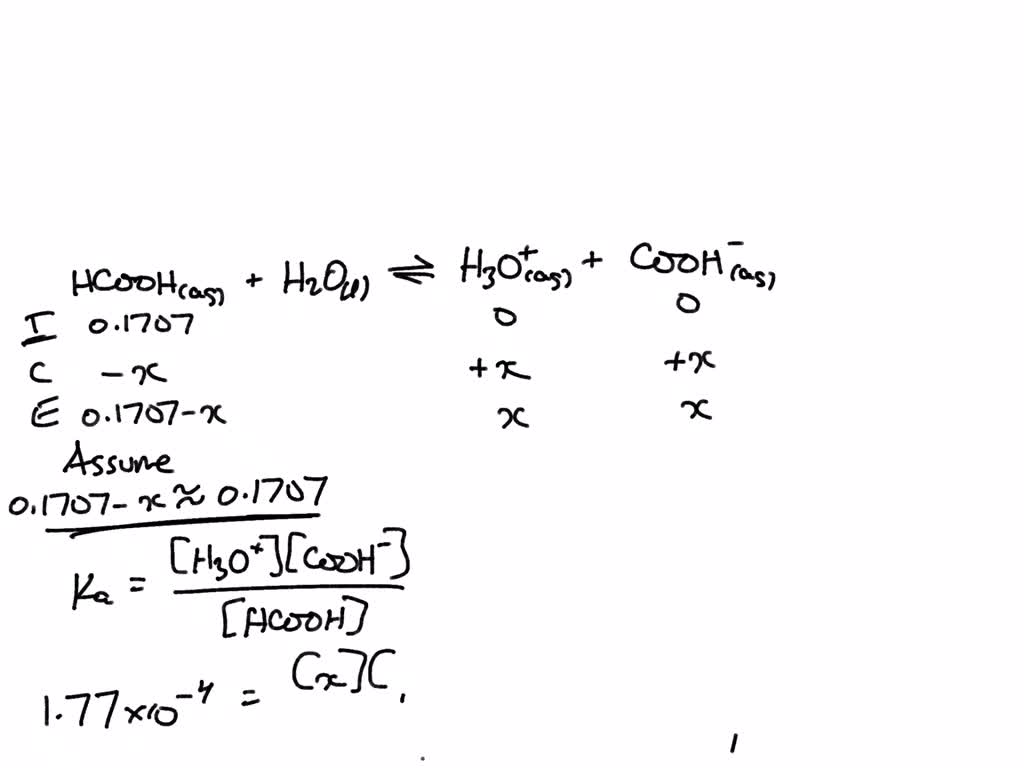
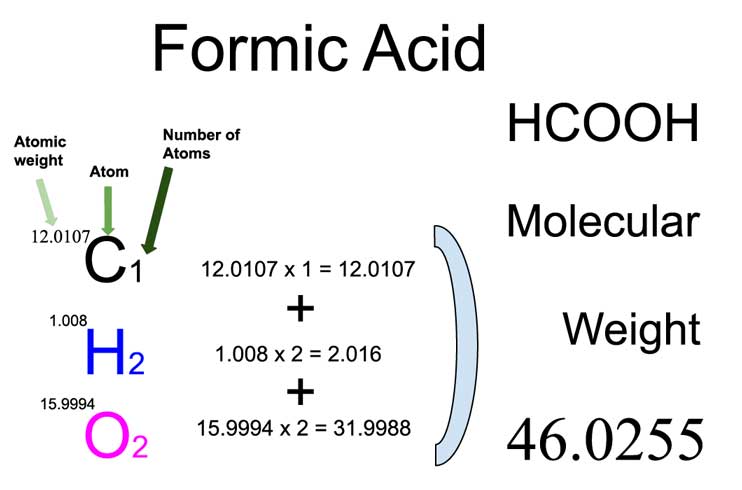
SOLVED: Formic acid is a weak acid with the formula HCOOH; the value of Ka for formic acid is 1.77 x 10-4 In aqueous solution, formic acid partially dissociates according to the

Energies | Free Full-Text | Cost Efficiency Analysis of H2 Production from Formic Acid by Molecular Catalysts

Dissociation constant (K_a) of formic acid and acetic acid are 2.5xx10^-4 and 0.5xx10^-5 respect... - YouTube
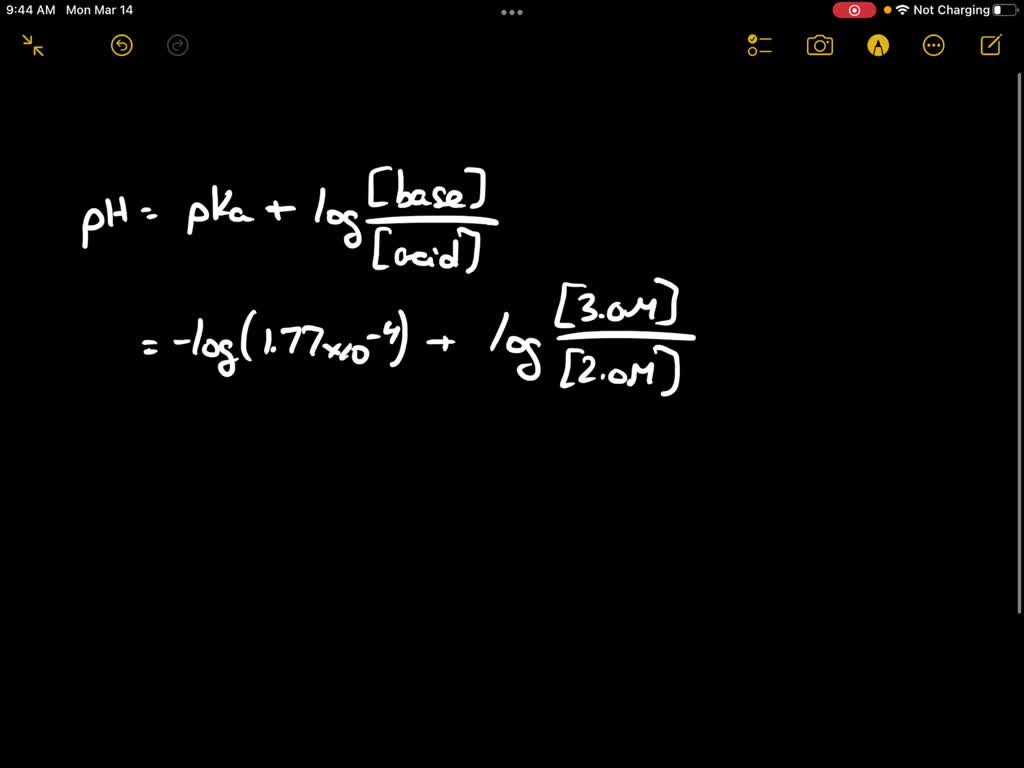
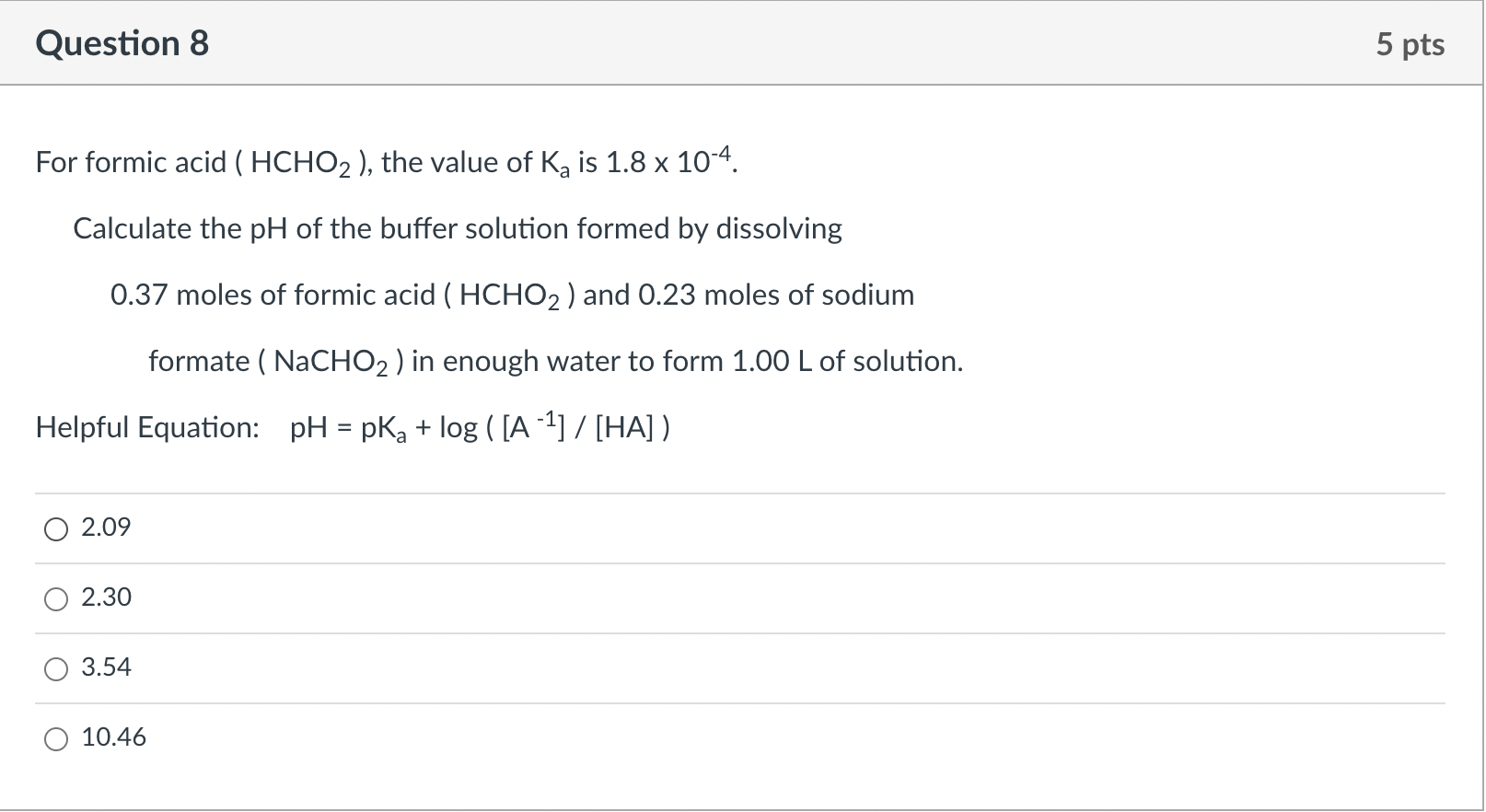
SOLVED: The Ka for formic acid, HCOOH, is 1.77 * 10^-4. HCOOH (aq) + H2O (l) ⇌ HCOO- (aq) + H3O+ (aq) What is the pH of a buffer made from 2.0

The Ka for formic acid is 18 x 10-4 What is the pH for a 035 M aqueous solution of sodium form - YouTube

Dehydrogenation of Formic Acid by a RuII Half Sandwich Catalyst - Vatsa - 2021 - ChemistrySelect - Wiley Online Library

When a solution of formic acid was titrated with KOH solution, the pH of the solution was 3.65 when half the acid was neutralized. Calculate Ka(HCOOH) .
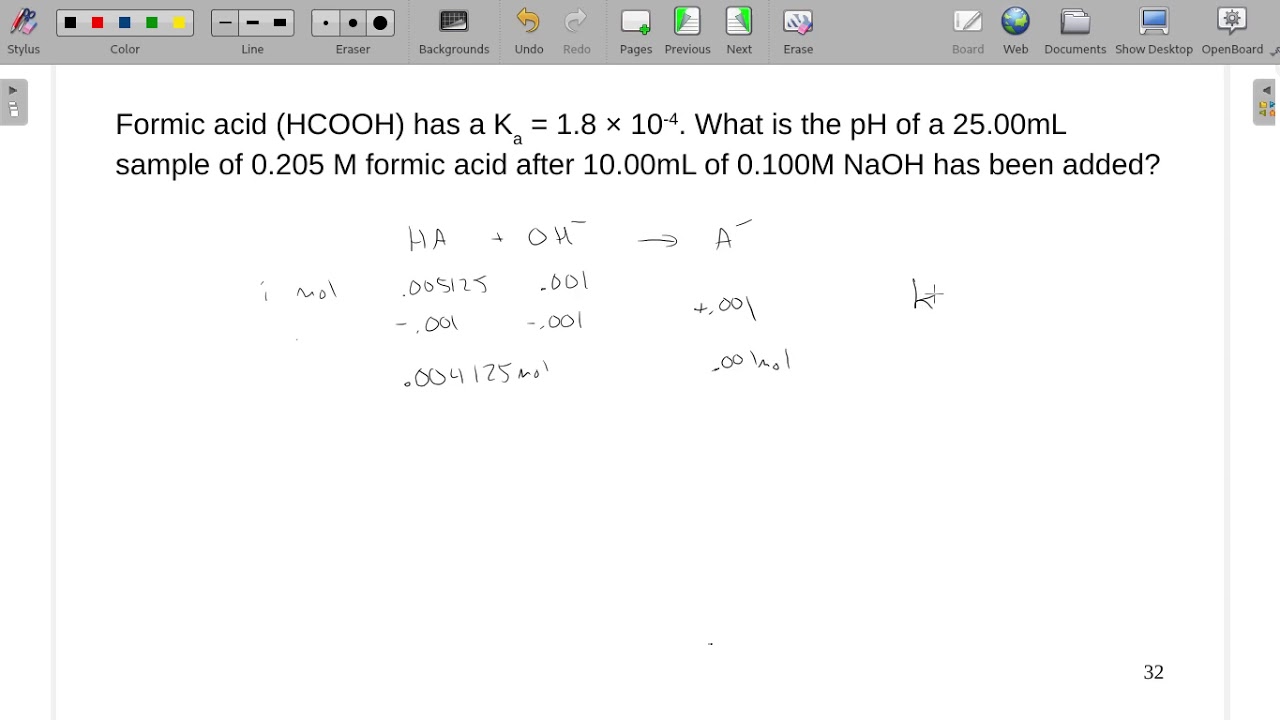
Formic acid has a Ka = 1.8×10-4. What is the pH of a 25.00mL sample of 0.205 M formic acid after... - YouTube

Carbon Dioxide Hydrogenation to Formic Acid with Self‐Separating Product and Recyclable Catalyst Phase - Ehmann - 2022 - ChemCatChem - Wiley Online Library
Formic acid, 50 ml, glass, 50 ml, CAS No. 64-18-6 | Starting material for eluent mixtures | Eluent additives for LC-MS | LC-MS | Liquid chromatography (LC, HPLC, LC-MS) | Chromatography | Applications | Carl Roth - International

Reaction and separation system for CO2 hydrogenation to formic acid catalyzed by iridium immobilized on solid phosphines under base-free condition - ScienceDirect










![a. A 0.1M solution of Formic acid [HCOOH] has Ka=1.77×10−4. Calculate (i).. a. A 0.1M solution of Formic acid [HCOOH] has Ka=1.77×10−4. Calculate (i)..](https://storage.googleapis.com/filo-classroom-notes/thumb_classroom_27777169_812PT.jpeg)