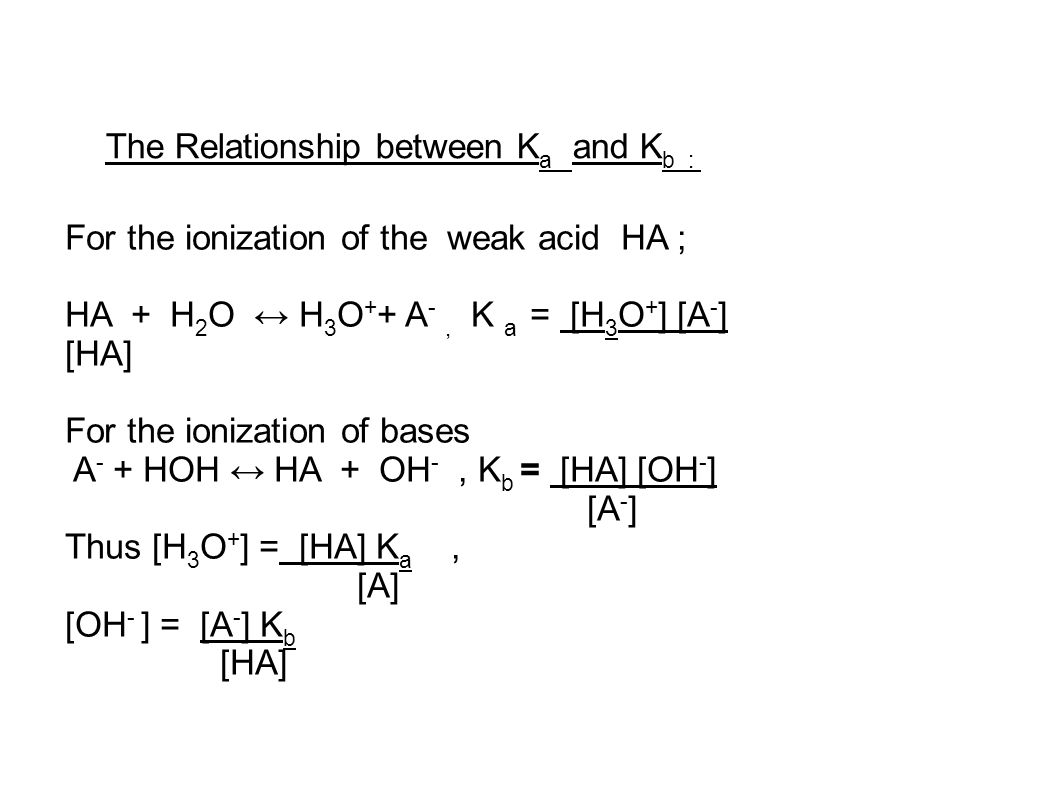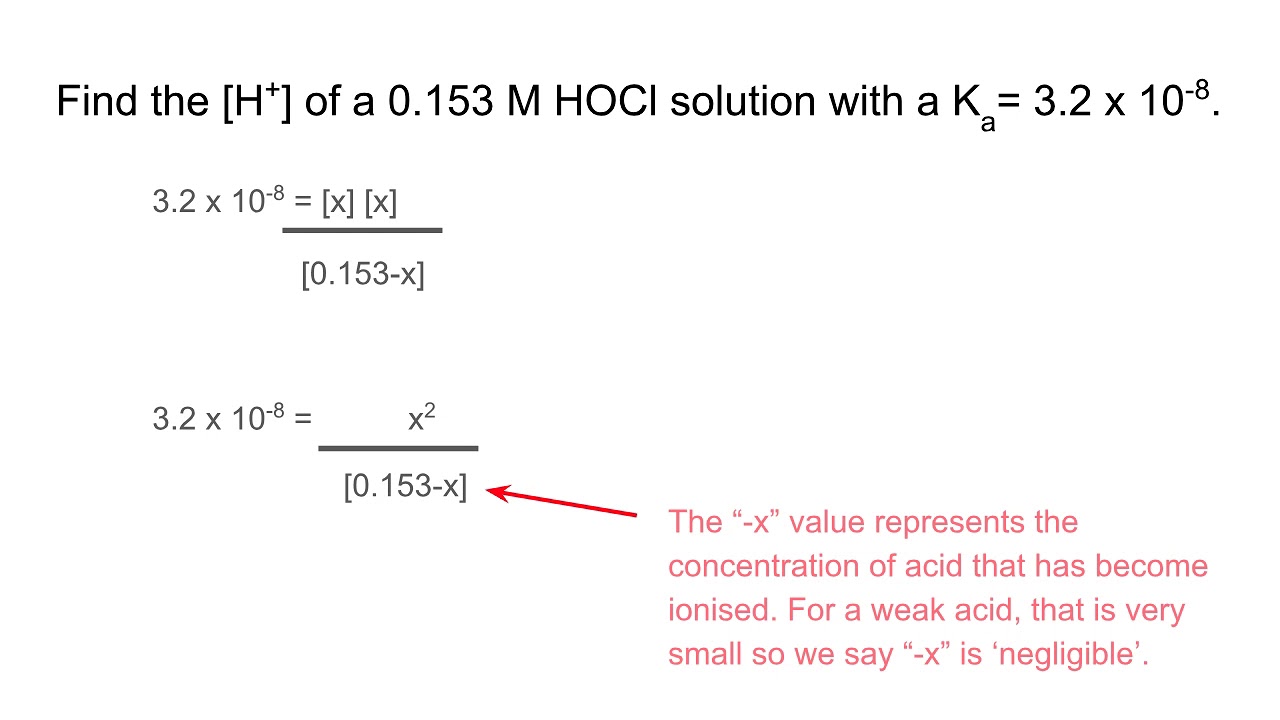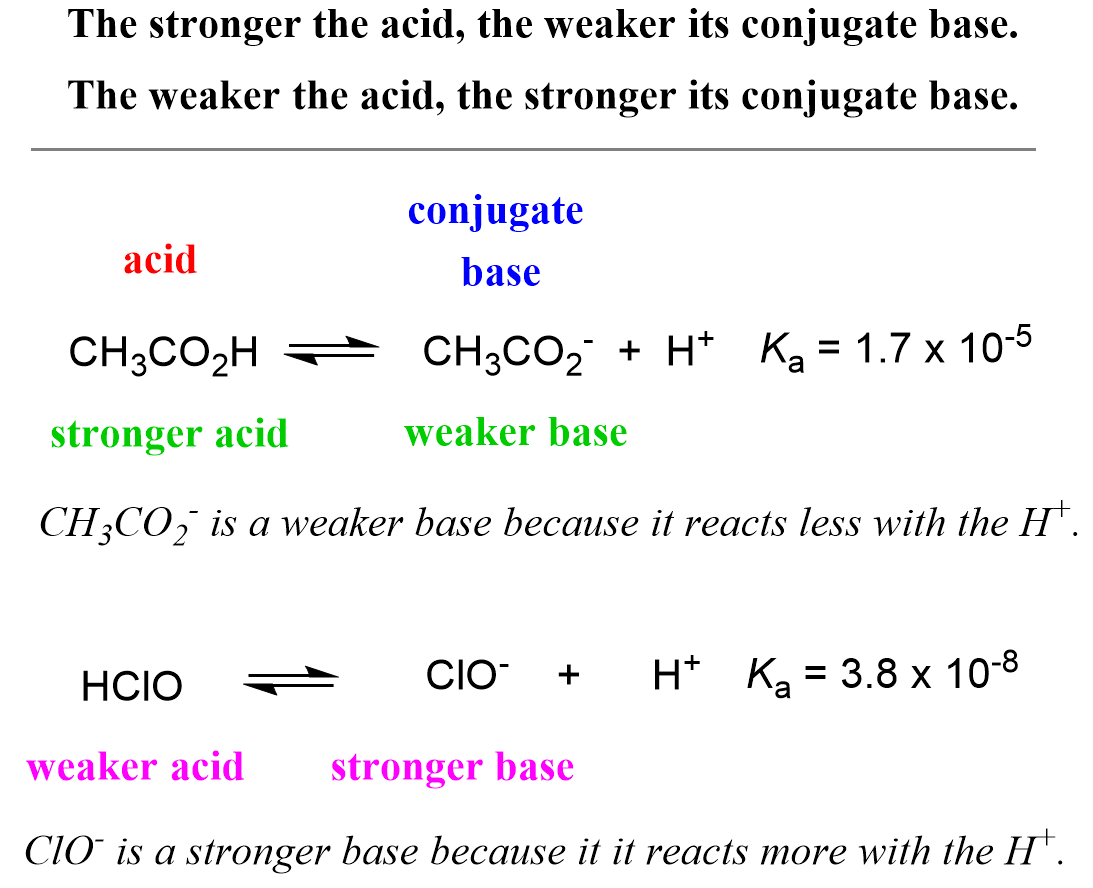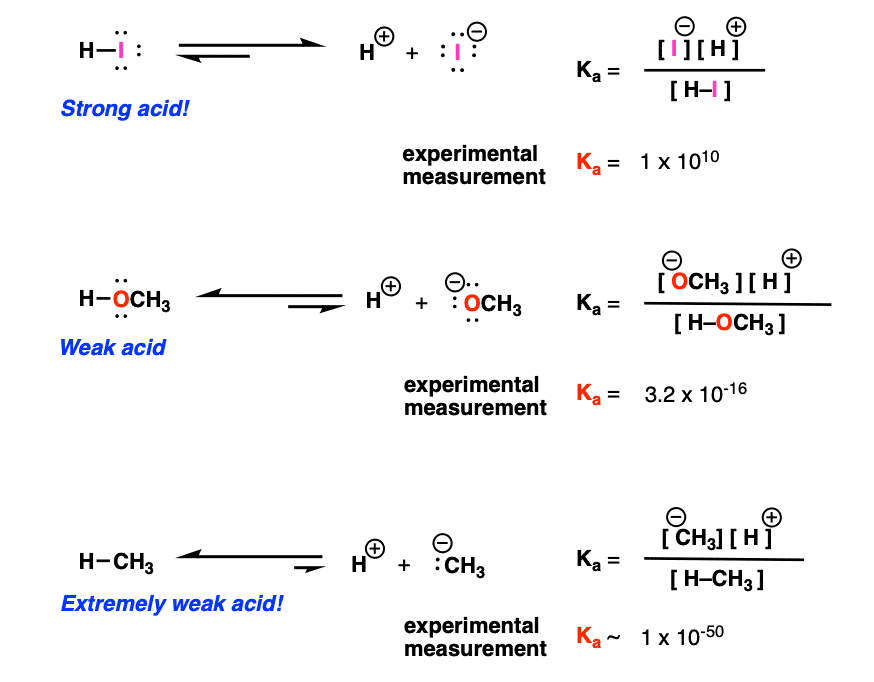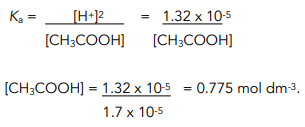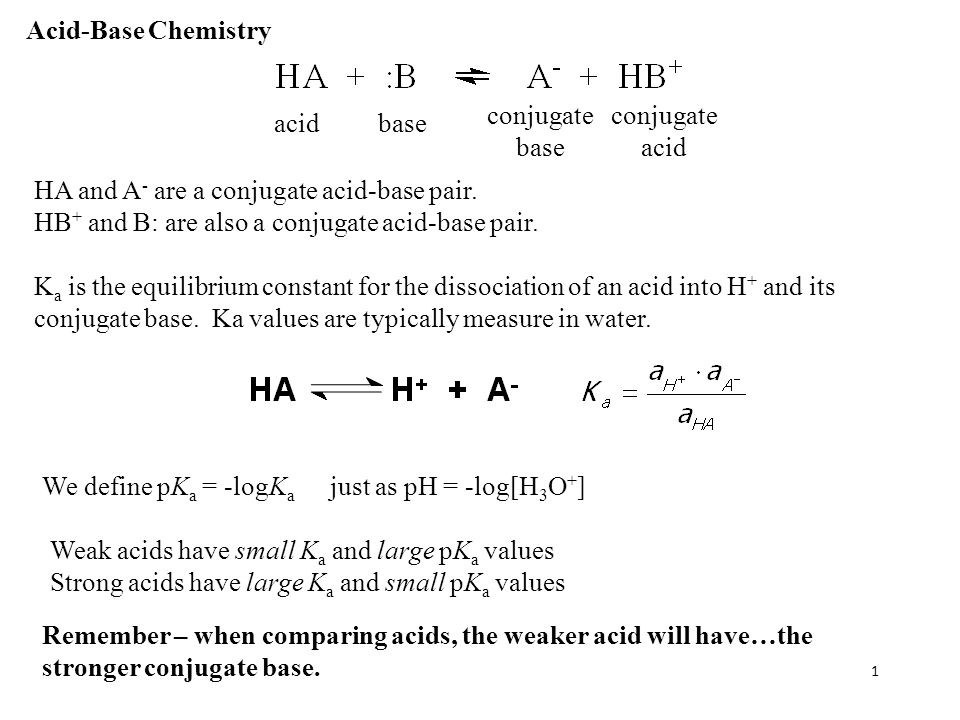
Acid-Base Chemistry K a is the equilibrium constant for the dissociation of an acid into H + and its conjugate base. Ka values are typically measure in. - ppt download
![Ka and Kb Calculations. For Weak Acid Reactions: HA + H 2 O H 3 O + + A - K a = [H 3 O + ][A - ] K a < 1 [HA] - ppt download Ka and Kb Calculations. For Weak Acid Reactions: HA + H 2 O H 3 O + + A - K a = [H 3 O + ][A - ] K a < 1 [HA] - ppt download](https://images.slideplayer.com/9/2507407/slides/slide_2.jpg)
Ka and Kb Calculations. For Weak Acid Reactions: HA + H 2 O H 3 O + + A - K a = [H 3 O + ][A - ] K a < 1 [HA] - ppt download

Question Video: Writing an Equation for the Acid Dissociation Constant of a Generic Weak Acid | Nagwa

Positive regulation of the enzymatic activity of gastric H+,K+-ATPase by sialylation of its β-subunit - ScienceDirect